Huaxiang Group, a leading medical 3D Printing solution provider, announced on Feb 11th, 2022 their reception of category 3 medical device clearance from China’s National Medical Products Administration (referred to as NMPA) for its Porous Titanium Interspinal Fusion Cages produced on Farsoon metal systems.
“Following the 3D printed porous titanium spine fusion cage – the very first approved orthopedic implants built by metal laser powder bed fusion technology in China in early 2021, we are proud to introduce this second NMPA approved implant device into our product portfolio.” Said Mr. Li Xinghua, head of marketing from Huaxiang Group: “this marks the beginning of the true application of metal 3D printing technology for spinal implant surgeries in China.”
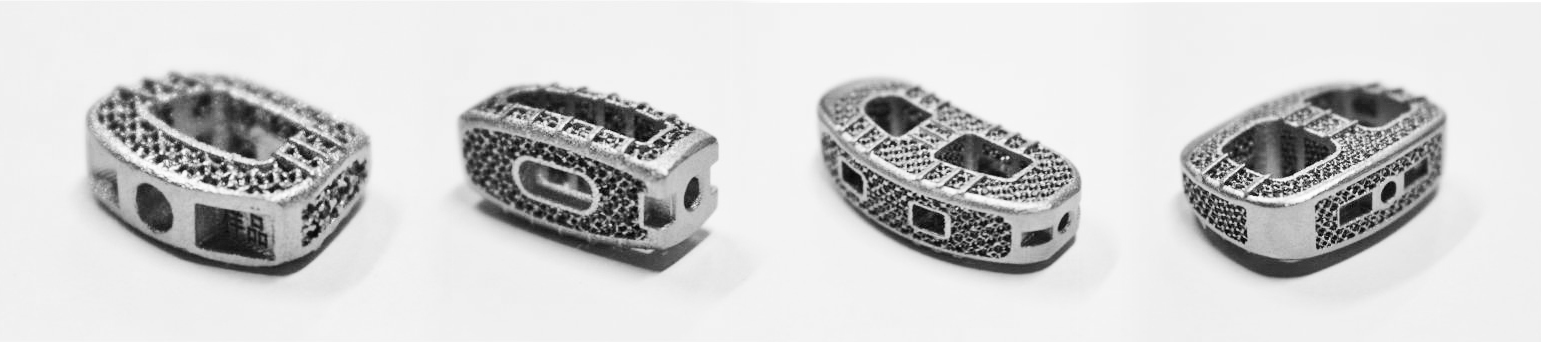
Figure 1: NMPA approved Porous Titanium Interspinal Fusion Cages 3D printed by Farsoon metal 3D printers. Image courtesy: Huaxiang Group.
With a fully patented technology, the Porous Titanium Interspinal Fusion cage is identified by the 3D Printing Technology Application of Personalized Implant Device project headed by the country’s leading hospitals, research institutes, material manufacturers and 3D printing specialists in the country. The medical grade additive manufacturing solutions jointly developed by Huaxiang Group and Farsoon – it showcases a combined knowledge in product development, medical-grade materials, additive manufacturing, quality control as well as clean-room environment monitoring.
Compared to conventional produced implants, Farsoon 3D printed Porous Interspinal Fusion Cage can be fully customized according to patients’ conditions, including the pore size, porosity and elastic modulus, to offer the most precise solution that helps promote bone growth rate. The as-built surface quality, mechanical properties and size accuracy can fully meet the highest standards for interspinal implants as well. The surgeons also see huge potential for implementing Farsoon metal 3D printing in the emergency surgeries with the significantly accelerated “on-demand” development and manufacturing process.
With years of technology development and field experiences in the medical industry, Farsoon and Huaxiang Group have introduced additive manufacturing to more than 80 Tertiary A hospitals, covering 16 clinical departments, and conducted more than 8,000 clinical cases across the country.
Farsoon strives to push the application of additive manufacturing in the medical field by working with industrial partners to offer high-quality, productive 3D printing solutions. During May 12-14th, 2022, Farsoon will be showcasing this Porous Interspinal Fusion Cage product at booth J19, Hall 4.1 at TCT Asia located in Shanghai National Convention and Exhibition Center. Inquires and interested customers are welcome to contact globalinfo@farsoon.com for more information.
About the 3D Printing Technology Application of Personalized Implant Device Project:
The 3D Printing Technology Application of Personalized Implant Device project is an initiative supported by the Ministry of Science and Technology in the “13th Five-Year Plan”, led by Shanghai Jiaotong University’s Ninth People’s Hospital, with a strong team consisting of members from the Chinese Academy of Sciences Institute of Metal Research, South China University of Technology, Sichuan University, Xi’an Jiaotong University, Shanghai Institute of Ceramics, Hubei University, Central South University, Farsoon Technologies and Huaxiang Group. The goal of the project is to establish, and extend the application of total 3D printing solutions for advanced medical devices including medical-grade material, 3D printing machines, design software, medical device development & clinical verification and medical supply chains.
About Huaxiang Group:
Huaxiang Group is the industrial leader of medical 3D printing application in China. With an established medical industrial chain including materials research and development, software, additive manufacturing, post processing, etc., Huaxiang Group is ready to offer hospitals and doctors with comprehensive, accurate and efficient industrial grade medical 3D printing solutions.
About Farsoon:
OPEN FOR INDUSTRY – Farsoon Technologies, founded in 2009, is a global manufacturer and supplier of industrial level polymer and metal laser sintering systems. Farsoon is the leading supplier of industrial AM technology in China with increasing growth in the international market. In 2017, Farsoon released the first of a new series of machines under the revolutionary Continuous Additive Manufacturing Solution (CAMS), and established Farsoon Technologies-Americas in Austin, Texas, USA. In 2018, Farsoon Europe GmbH was established in Stuttgart, Germany to expand direct operations to Europe. Farsoon is committed to developing AM towards its true manufacturing potential and providing customers with best-in-class systems, materials, software, applications and services. Learn more: www.farsoon.com